DAC INFO is dedicated to implementing the concepts for Computer System Validation as per Good Automated Manufacturing Practices and helping companies address the requirements of GAMP 5, Annex 11, and 21 CFR Part 11 Electronic Records and Electronic Signatures.
DAC INFO is a wide spectrum IT company offering a broad range of computerized system validation services, also the latest automation solutions to Life Sciences verticals such as pharmaceutical, biotechnology pharmaceutical, s, medical equipment manufacturing, genetics research. Our skills extend from setting up compliant and validated systems to custom CRM and other software development. We offer engineering-related services such as full project automation in turnkey electrical systems, on-site installation, systems upgrade for automation, and energy management.
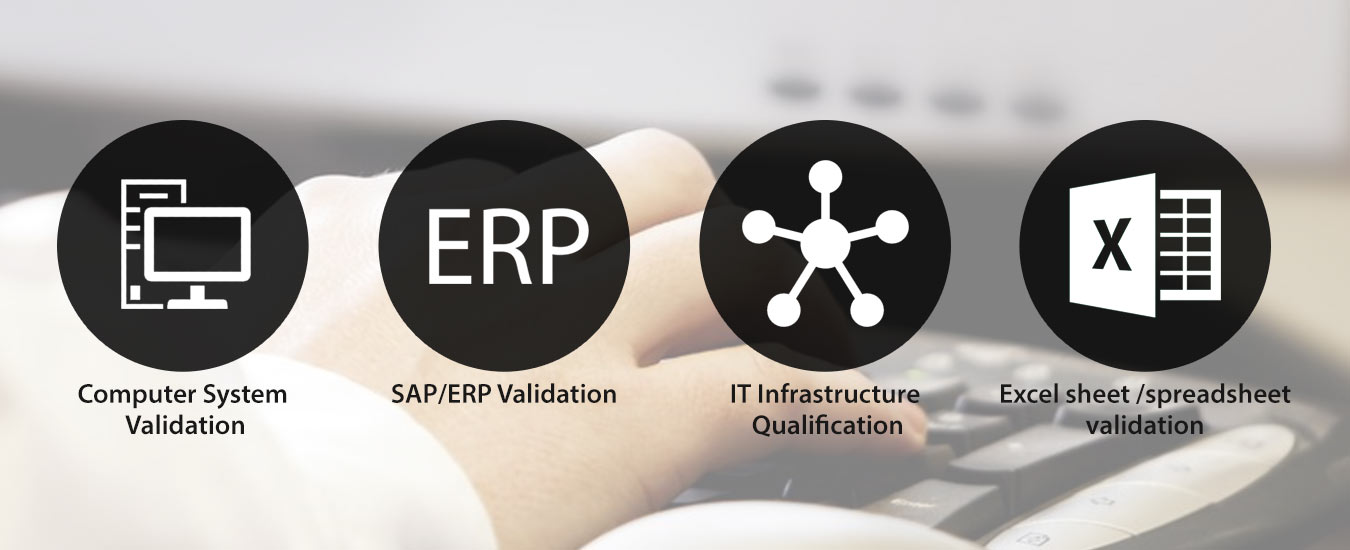
Adequate compliance protocols are imperative to integrate with the standard global IT framework. The alignment with the network is essential for businesses to grow in a language of cooperation accepted as standard. We deliver services in the areas of SAP/ERP validation, spreadsheet validation, PLC validation, IT infrastructure qualification, and computer system validation.
Our software and web development team can create brilliant digital protocols to meet your unique standards. Whether you need a special CRM solution or a full-fledged e-commerce website, we are always up to the task. We also develop applications for mobile platforms and handle the Search Engine Optimization requirements for our clients’ websites. We also offer excellent web development and mobile app development services.